Platelets and Clotting: 7 Powerful Facts to Know
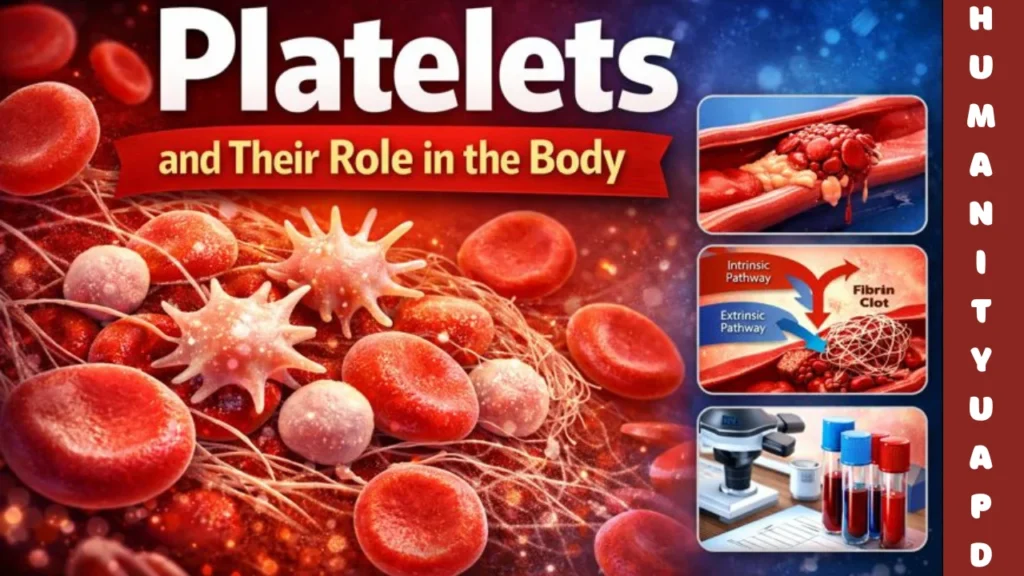
Platelets and Clotting
Platelets, also known as thrombocytes, are a crucial component of the blood, playing a significant role in the body’s ability to maintain hemostasis. These small, disc-shaped cell fragments are produced in the bone marrow from larger cells known as megakaryocytes. Although platelets are often referred to as cells, they are actually cell fragments and lack a nucleus, but they are vital for proper blood function.
The primary role of platelets is in the clotting process, which is essential for preventing excessive bleeding when injuries occur. When a blood vessel is damaged, platelets quickly migrate to the site of injury and adhere to the exposed collagen fibers. This initial response is part of a complex process that also involves other cellular components and signaling molecules. Platelet activation leads them to undergo a transformation where they change shape and release chemical signals, facilitating further aggregation and the recruitment of more platelets to the injured area.
This aggregation forms a temporary plug that helps seal the wound, a process referred to as primary hemostasis. Moreover, platelets release various substances, such as clotting factors and growth factors, which not only assist in the formation of a stable blood clot but also play a vital role in tissue healing and repair. The overall functionality of platelets is critical, as an imbalance in their number or activity may lead to disorders such as excessive bleeding or unwanted blood clot formation, both of which can have serious health implications.
In essence, understanding platelets and their functions is important for appreciating how the body responds to injuries and maintains vascular integrity. This overview sets the stage for further exploration into the intricacies of the clotting process and the broader implications of platelet dynamics for human health.
👉 Table of Contents 👇
The Biology of Platelets: Structure and Function
Platelets, also known as thrombocytes, are critical components of the blood involved in hemostasis, the process that prevents excessive bleeding when injuries occur. These small, disc-shaped cell fragments are derived from megakaryocytes in the bone marrow. Understanding their structure is essential to fully grasping their vital role in the clotting process.
The structure of platelets includes several key components. One of the most important is the plasma membrane, which is responsible for maintaining the integrity of the platelet while also facilitating the interaction with other cells and platelets. Embedded within this membrane are various receptors that allow platelets to communicate with one another and respond to signaling molecules involved in the clotting cascade.
Granules are another significant component of platelets, providing them with the necessary substances needed during clot formation. Platelets contain two types of granules: alpha granules and dense granules. Alpha granules store proteins such as fibrinogen and von Willebrand factor, which are crucial for clot formation and stability. Dense granules, on the other hand, contain substances like ADP and calcium ions, which help to activate other platelets and promote the aggregation process.
Moreover, the cytoskeletal elements of platelets, primarily actin and myosin, grant them the ability to change shape during activation. Upon injury to a blood vessel, platelets undergo a dramatic change, becoming activated and spreading out to form a plug. This process is facilitated by the interplay between their structural components and signaling pathways initiated by vascular injury.
In summary, the intricate structure of platelets encompasses various components that work together harmoniously. Their origin from bone marrow, combined with their granules, receptors, and cytoskeletal framework, enables platelets to effectively contribute to the clotting process and protect the body from excessive blood loss.
The clotting cascade is a complex series of events that plays a crucial role in hemostasis, the process that prevents excessive bleeding when vascular injury occurs. It comprises two primary pathways: the intrinsic and the extrinsic pathways. Both pathways converge on a common pathway that ultimately leads to the formation of a stable blood clot. Platelets, along with various clotting factors, are integral to this process.
The intrinsic pathway is initiated by damage to the blood vessel wall, exposing subendothelial collagen. Platelets adhere to these exposed areas, becoming activated and releasing a cascade of signals. This activation leads to further recruitment of platelets and the activation of clotting factors, such as Factor XII, which activates Factor XI, and in turn, activates Factor IX. The activation continues with Factor IX forming a complex with Factor VIII, resulting in the activation of Factor X. This step is critical, as it bridges the intrinsic pathway to the common pathway.
Conversely, the extrinsic pathway is triggered by external trauma, such as cuts or lacerations. Tissue factor (TF), released from damaged endothelial cells, interacts with Factor VII, leading to the activation of Factor VIIa. The activated Factor VIIa then catalyzes the activation of Factor X. The coagulation cascade rapidly accelerates during this process, underscoring the importance of both pathways in hemostasis.
Once Factor X is activated, it converts prothrombin to thrombin in the common pathway. Thrombin is a pivotal enzyme that facilitates further platelet activation, borrowing from the previously activated platelets, while promoting the conversion of fibrinogen to fibrin strands. These strands weave through the aggregated platelets, stabilizing the complex and effectively forming a blood clot. This interplay between platelets and clotting factors ensures the rapid formation of a hemostatic plug, a crucial step in preventing blood loss.

Platelet Activation: Triggers and Processes
Platelet activation is a crucial step in hemostasis, the process that prevents excessive bleeding when blood vessels are injured. This activation occurs in response to various triggers, predominantly vascular injury. When the endothelial layer of blood vessels is disrupted, it exposes underlying collagen and other subendothelial components, which are potent activators of platelets. Shortly after injury, platelets adhere to these exposed sites, initiating a cascade of reactions essential for clot formation.
Upon adhering to the site of injury, platelets undergo a significant morphological change known as shape change, transitioning from a disc-like structure to a more spherical shape with numerous extensions. This transformation not only increases the surface area of the platelets but also enhances their ability to interact with one another and with other components of the hemostatic system.
Additionally, platelet activation is accompanied by the release of granules, which contain various bioactive substances such as adenosine diphosphate (ADP), thromboxane A2, and serotonin. These substances play pivotal roles in promoting further platelet activation and recruitment to the site of injury. The granule release triggers a feedback loop that amplifies the activation process, encouraging more platelets to aggregate.
Aggregation occurs as activated platelets express glycoprotein receptors on their surface, which bind fibrinogen and other adhesive proteins. This leads to platelet clustering, forming a platelet plug that serves as a temporary solution to vascular injury. Together, these processes—triggered by vascular injury, marked by shape change, granule release, and subsequent aggregation—play a vital role in the hemostatic process, ensuring rapid response to vascular damage and preventing blood loss.
The Role of Platelets in Wound Healing and Inflammation
Platelets, primarily known for their critical function in hemostasis, play an essential role in wound healing and the inflammatory response. Beyond their ability to form clots, platelets release a myriad of growth factors and cytokines that are pivotal in tissue repair. When an injury occurs, platelets are among the first responders. They adhere to the exposed collagen fibers at the site of injury, activating their complex signaling pathways.
The activation of platelets triggers the release of several growth factors such as platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β). These factors promote the proliferation and migration of various cell types, including fibroblasts and endothelial cells, which are crucial for tissue regeneration. By enhancing angiogenesis, the process of new blood vessel formation, platelets facilitate the delivery of oxygen and nutrients necessary for healing.
In addition to their reparative functions, platelets play a significant role in the inflammatory response. Upon activation, they release pro-inflammatory cytokines that help recruit leukocytes to the site of injury. This recruitment is critical, as leukocytes, especially neutrophils and monocytes, contribute to the clearance of pathogens and debris, thereby preventing infection. Furthermore, platelets also assist in modulating the inflammatory response, balancing the pro-inflammatory signals with anti-inflammatory factors to ensure that healing progresses without excessive tissue damage.
Moreover, recent research has unveiled that platelets contribute to the formation of biofilms and the modulation of immune responses, showcasing their multifaceted role in both wound healing and inflammation. Their participation in these processes underscores the importance of platelets not just in clotting but as integral players in the body’s healing and defense mechanisms.
Platelet Disorders: Thrombocytopenia and Thrombocythemia
Platelets are essential components of the blood, playing a critical role in hemostasis—the process that prevents excessive bleeding. However, certain disorders can disrupt their normal function and levels, leading to conditions such as thrombocytopenia and thrombocythemia.
Thrombocytopenia is characterized by a low platelet count, often defined as fewer than 150,000 platelets per microliter of blood. This condition can result from various factors, including bone marrow disorders, certain medications, viral infections, and autoimmune diseases. Individuals with thrombocytopenia may experience symptoms such as easy bruising, prolonged bleeding from cuts, and petechiae, which are small red or purple spots on the skin. Diagnosis typically involves blood tests to evaluate platelet counts and underlying causes. Treatment options aim to address the underlying issue and may include corticosteroids to suppress the immune system, platelet transfusions in severe cases, or medications to stimulate platelet production.
On the other hand, thrombocythemia is marked by an elevated platelet count, generally over 450,000 platelets per microliter. This disorder may be primary, resulting from a myeloproliferative neoplasm—where the bone marrow produces too many platelets—or secondary due to other conditions such as chronic inflammation or iron deficiency. Those affected may be asymptomatic or experience complications such as increased risk of thrombosis, where blood clots form in blood vessels. Symptoms might include headaches, dizziness, and vision disturbances. Management of thrombocythemia often focuses on reducing the risk of clotting events and may include medications such as aspirin or more aggressive treatments like bloodletting or chemotherapy in severe cases.
Understanding these platelet disorders is crucial in managing patient care effectively. Awareness of the symptoms, causes, and treatment options can significantly improve outcomes for those affected by thrombocytopenia and thrombocythemia.
Diagnostic Tests for Platelet Function and Count
Understanding platelet function and count is essential in diagnosing and managing various bleeding disorders and thrombotic conditions. A variety of diagnostic tests are utilized in clinical practice to evaluate the functionality of platelets and their quantity, each providing valuable insights into an individual’s hemostatic profile.
One of the most fundamental tests is the complete blood count (CBC), which measures the overall platelet count in the blood. This test provides an initial screening for conditions like thrombocytopenia (low platelet count) and thrombocytosis (high platelet count). Abnormal results in a CBC can prompt further investigation to determine the underlying causes, which may range from bone marrow disorders to liver disease.
Another significant test is platelet aggregometry, which assesses the ability of platelets to aggregate or clump together in response to various agonists, such as adenosine diphosphate (ADP) or collagen. This test is crucial for identifying inherited or acquired platelet function disorders, such as Glanzmann thrombasthenia or von Willebrand disease. By evaluating how well platelets respond to these stimuli, clinicians can understand the functional integrity of the platelet population.
Flow cytometry is also increasingly used to assess platelet function. This advanced technique can identify and measure specific surface markers on platelets, providing detailed information about their activation and response to various stimuli. Additionally, platelet function tests may also include the evaluation of platelet response to antiplatelet medications, which is particularly important in patients undergoing antithrombotic therapy.
Each of these tests plays a pivotal role in diagnosing and managing bleeding disorders, informing treatment decisions, and monitoring therapeutic effectiveness. Clinicians often select a combination of tests based on clinical suspicion, patient history, and the need for comprehensive assessment, ensuring accurate diagnosis and optimal patient care.

Advances in Platelet Research and Future Directions
Recent advancements in platelet research have opened new avenues for the development of therapies targeting platelet function, especially in the context of bleeding disorders and cardiovascular diseases. Scientists have been focusing on understanding the complex biology of platelets, their role in hemostasis, and the molecular mechanisms that regulate their function. These insights are pivotal in identifying new therapeutic targets.
One major area of advancement is the study of platelet activation pathways. Researchers have identified specific receptors and signaling pathways that play key roles in platelet activation and aggregation. For instance, understanding the signaling cascade triggered by the glycoprotein receptors on the surface of platelets has led to the exploration of novel inhibitors that can specifically disrupt these pathways. Such inhibitors could effectively reduce thrombus formation in patients prone to cardiovascular events while minimizing the risk of bleeding.
Another significant development is the exploration of platelet-derived extracellular vesicles and their function in intercellular communication within the vascular system. These vesicles have been shown to carry bioactive molecules, influencing not only platelet function but also endothelial cell behavior and inflammation. Targeting these vesicles may provide innovative therapeutic strategies to modulate platelet activity in various pathological conditions.
Furthermore, advancements in biotechnology have enabled the identification of biomarkers associated with platelet activation. These biomarkers hold promise for the early detection of thrombotic and bleeding disorders, providing an opportunity for timely intervention. As research progresses, it is anticipated that personalized approaches based on patient-specific platelet function profiles could enhance treatment outcomes in conditions such as anticoagulation therapy or in the management of individuals with bleeding disorders.
As researchers continue to unveil the complexities of platelet biology, the potential for novel therapies targeting platelet function appears promising. Future endeavors will likely focus on integrating multidisciplinary approaches, combining insights from molecular biology, pharmacology, and clinical sciences to bring these innovative treatment strategies to fruition.
FAQs about Platelets and Clotting
Understanding platelets and their role in the clotting process is essential for both medical personnel and the general public. Below are some frequently asked questions that shed light on these vital components of blood.
What are platelets?
Platelets, or thrombocytes, are small, disc-shaped cell fragments in the blood that play a crucial role in hemostasis, the process of blood clotting. They are produced in the bone marrow and are essential in preventing excessive bleeding following injury.
How do platelets contribute to clotting?
Upon vascular injury, platelets are activated and begin to adhere to the exposed collagen of the damaged vessel. This activation triggers a series of responses, leading to the release of signaling molecules that amplify platelet activation. The platelets then aggregate, forming a soft plug that obstructs blood flow. Subsequently, a cascade of coagulation factors leads to the formation of fibrin, solidifying the clot.
What are some common platelet disorders?
Platelet disorders can generally be classified into two categories: quantitative or qualitative. Thrombocytopenia is a condition characterized by a low platelet count, which can lead to increased bleeding. Conversely, thrombocythemia is involving an excessive number of platelets. Qualitative disorders, such as Glanzmann’s thrombasthenia, involve dysfunctional platelets that are unable to perform their clotting functions effectively. Both types can present significant health concerns, emphasizing the importance of platelet function in clotting.
Can platelets affect cardiovascular health?
Indeed, abnormal platelet activation can contribute to thrombotic disorders, such as heart attacks and strokes. Ensuring normal platelet function is thus vital for cardiovascular health, as both hypo- and hyperactivity may lead to serious complications.
This overview of frequently asked questions helps clarify the vital role of platelets in the clotting process and addresses common misconceptions surrounding them.

Discover more from HUMANITYUAPD
Subscribe to get the latest posts sent to your email.

